MILD Procedure
Minimally Invasive Lumbar Decompression (mILD®)
The mild® Procedure is an FDA-cleared minimally invasive treatment option that addresses a major root cause of lumbar spinal stenosis (LSS). LSS is primarily a degenerative, age-related narrowing of the lower spinal canal that causes symptoms of pain, numbness, tingling or heaviness when standing or walking in the low back, legs, or buttocks. The mild® Procedure treats this condition by removing excess, thickened ligament tissue to restore space in the spinal canal and reduce the pressure on the spinal nerves.
mild® is an early treatment option to consider when conservative therapies such as physical therapy, pain medication, and chiropractic treatments do not provide adequate relief. The short, outpatient mild® Procedure can be performed through a single, tiny incision smaller than the size of a baby aspirin (5.1 mm) and is covered by Medicare and Medicare Advantage plans nationwide.
Minimally invasive lumbar decompression, commonly referred to as the mild® Procedure, is an outpatient procedure that relieves pressure on the spine. Like a water hose that has pressure on it or a drinking straw with a kink in it, the spinal canal can narrow and compress the spinal canal nerves in the lower back. To restore space in the spinal canal and reduce the compression of the nerves—or in the case of the water hose and drinking straw, increase the flow—a mild® Doctor uses an imaging machine and specialized tools to remove small pieces of bone and thickened ligament.
The mild® Procedure is different from traditional back surgery because it is an outpatient procedure that does not require general anesthesia, implants, stitches, steroids or opioids. Typically completed in less than an hour, mild® can be performed through a single, tiny incision smaller than the size of a baby aspirin (5.1 mm) using only local anesthetic and light sedation and leaves no implants behind. With a safety profile similar to an epidural steroid injection (ESI), but with lasting results, mild®addresses a major root cause of lumbar spinal stenosis (LSS) and can be performed as a steroid-free procedure, which limits immunosuppression risks associated with ESIs.
BENEFITS
mild® may help patients diagnosed with lumbar spinal stenosis (LSS) stand longer and walk farther with less pain by treating the underlying cause of LSS symptoms in a minimally invasive way.
Benefits of the mild® Procedure
- Outpatient procedure, typically performed in less than 1 hour
- Patients typically resume normal activity within 24 hours with no restrictions
- Requires no general anesthesia, implants, stitches, steroids or opioids
- Low complication risk profile and high efficacy profile as demonstrated in clinical studies
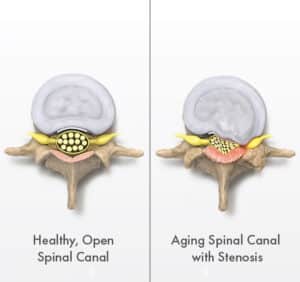
About Lumbar Spinal Stenosis (LSS)
WHAT IS LSS?
- A condition in which the lower spinal canal narrows and compresses the spinal nerves in the lower back.
- Best visualized as a “kink in a drinking straw,” this compression can contribute to pain and mobility issues.

HOW COMMON IS LSS?
- Over two million LSS patients
nationwide are diagnosed and treated annually3 - Generally found in people over the age of 50
- The likelihood of developing LSS increases with age
WHAT ARE THE CAUSES OF LSS?
The natural wear and tear on the spine as people age can lead to a number of contributing factors that cause the narrowing of the spinal canal and create pressure on the spinal nerves:
- Thickening of ligament tissue
- Overgrowth of bone (osteoarthritis)
- Bulging of discs
HOW IS LSS DIAGNOSED?
In addition to taking a medical history that includes a list of your symptoms, other tests may be performed to verify LSS:
- Physical examinations to test mobility
- Imaging: X-rays, MRI, CT scan
Symptoms of LSS
- Pain or numbness in the lower back when standing upright
- Pain, numbness, heaviness or tingling in upper legs or buttocks when walking
- Pain when lying down that may be relieved by curling into the fetal position
- Temporary relief when bending forward while sitting or standing as pressure on the spinal cord is released and space in the spinal canal is “opened”
- Common actions include finding a chair, leaning over shopping cart, using walker or cane
LSS Treatment Options
mild® is an early treatment option to consider when conservative therapies (e.g., physical therapy, pain medication, chiropractic) are not providing adequate relief. The mild® Procedure addresses a major root cause of LSS by removing excess ligament tissue to restore space in the spinal canal. The mild® Procedure typically takes less than an hour and can be performed through a single, tiny incision smaller than the size of a baby aspirin (5.1 mm).
Clinical Outcomes for mild®
INCREASE STANDING TIME
Cleveland Clinic 1-Year Study: Patients were able to increase their average standing time from 8 minutes to 56 minutes with less pain.
INCREASE WALKING DISTANCE
Cleveland Clinic 1-Year Study: Patients were able to increase their average walking distance from 246 feet to 3,956 feet with less pain.
AVOID BACK SURGERY
Cleveland Clinic 5-Year Study: mild® helped 88% of patients avoid back surgery for at least 5 years while providing lasting relief.
SAFETY PROFILE SIMILAR TO AN EPIDURAL STEROID INJECTION
MiDAS ENCORE 2-Year Study: mild® has a safety profile similar to an epidural steroid injection, but with lasting results. In this study, there were no statistically significant differences in the safety profile between study groups.
mild® is a minimally invasive procedure typically performed in an outpatient setting using only local anesthetic and light sedation. The nature of the procedure carries risks that do not apply to receiving an epidural steroid injection.
PATIENT SATISFACTION
MiDAS ENCORE 2-Year Study: Patients reported an 85% patient satisfaction rate with the mild® Procedure.
Strong Safety Profile
SAFETY PROFILE SIMILAR TO AN EPIDURAL STEROID INJECTION
- mild® has a safety profile similar to an epidural steroid injection, but with lasting results
MINIMALLY INVASIVE OUTPATIENT PROCEDURE
- Incision smaller than the size of a baby aspirin. No implants left behind, only a Band-Aid.
- Can be performed using local anesthetic and light sedation
FDA-CLEARED
- mild® is cleared by the U.S. Food and Drug Administration for lumbar decompressive procedures
MAINTAINS STRUCTURAL ANATOMY
- mild® does not significantly alter the structural anatomy of the spine
LOW COMPLICATION RATE
- Complication rate <0.1% in all commercial cases.
- No device- or procedure-related serious complications reported in any clinical trial.
- Compatible for patients with existing risk factors.
Frequently Asked Questions From Patients
Does the mild® Procedure require general anesthesia?
No, the mild® Procedure is performed in an outpatient setting and does not require general anesthesia. The mild® Procedure is generally performed with only local anesthetic and light sedation.
How long does the mild® Procedure take?
mild® is a short outpatient procedure that is typically performed in less than one hour. Most patients return home the same day and typically resume normal activity within 24 hours with no restrictions.
Is mild® accepted by my insurance?
The mild® Procedure is covered nationwide by Medicare (all ages, all plan types, including Medicare Advantage) the VA, U.S. Military & IHS. Commercial coverage varies. Talk to your doctor to obtain coverage specifics for your plan type.
What are the side effects of the procedure?
The mild® Procedure has a strong safety profile and has been performed on thousands of patients. Individual results may vary.
What is the recovery time like?
Following the mild® Procedure, most patients return home the same day and typically resume normal activity within 24 hours with no restrictions.
Is mild® cleared by the FDA?
Yes, the mild® Device kit was cleared by the U.S. Food and Drug Administration for lumbar decompressive procedures.