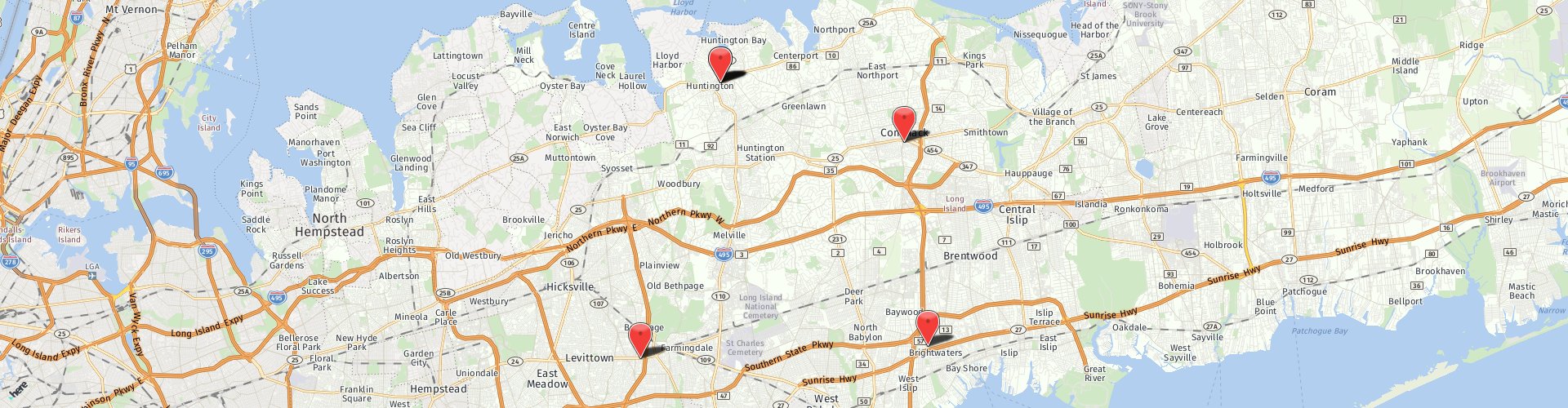
SpineJack
For decades, osteoporotic vertebral compression fracture (VCF) treatment has focused primarily on pain management. SpineJack is far different because it gives doctors the tools to restore vertebral body height, so your anatomy can be close to what it was before the fracture.
Benefits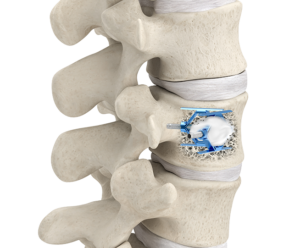
- Restores mid-vertebral body height
- Demonstrates improved pain scores in patients at 1 month and 6 months following the procedure compared to BKP treatment
- Protects from adjacent level fractures
Indications
The Stryker SpineJack product is used to treat pathological fractures of the vertebral body due to osteoporosis, cancer, or benign lesions. Cancer includes multiple myeloma and metastatic lesions, including those arising from breast or lung cancer, or lymphoma. Benign lesions include hemangioma and giant cell tumor.
Details
Control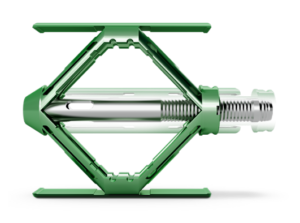
Unlike a balloon that expands according to the path of least resistance, the SpineJack system allows you to take control of implant placement and expansion in a craniocaudal direction.
Restore
Restoring anatomy is paramount when it comes to fracture treatment, and the SpineJack system maximizes every millimeter. The SpineJack system gives you the tools and the confidence to restore vertebral body height like never before.
The SpineJack system protects against adjacent level fractures, which may occur due to changes in the load distributed to adjacent vertebral bodies after an osteoporotic vertebral compression fracture.
Procedure
Before the procedure
Your doctor will do a physical exam and order imaging tests, such as an X-ray, MRI, CT scan or bone scan. These tests help to determine the location of the fractured vertebra, how recently the fracture occurred and whether or not vertebral augmentation with the SpineJack implant is the most appropriate treatment.
During the procedure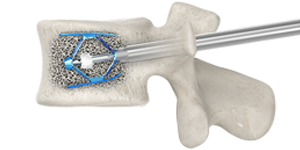
Generally, vertebral augmentation is performed while you are awake but sedated and your back is numbed with local anesthetic. Using X-ray guidance, two expandable implants are inserted into the fractured vertebra through small incisions. The implants are then expanded, restoring the vertebral anatomy and creating a cavity. The area surrounding the implants is then filled with bone cement to stabilize the fracture. As it hardens, the bone cement forms an internal cast that holds the vertebra in place. Following the procedure, the incisions are covered with bandages.
After the procedure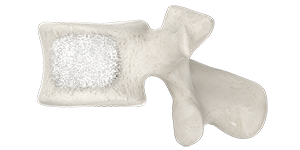
For a short period of time, you’ll lie on your back while the bone cement continues to harden. Your vital signs will be monitored. Typically, patients are able to go home within a few hours of treatment.
Risks and complications
Serious side effects have been known to occur with the use of bone cement in surgical procedures of the spine. These include heart attack, cardiac arrest (heart stops beating), stroke, embolism (blood clot or bone cement that moves to the heart or lungs) or death. Side effects may occur up to one year after the procedure.
Side effects related to use of the SpineJack system with bone cement include infection, bleeding, allergic reaction, thrombosis (blood clot formation), numbness or tingling and changes in blood pressure. Please consult with your doctor for the full list of possible side effects related to the combined use of bone cement with the SpineJack implant.
Credits & Sources:
- Nguyen U et al. Increasing prevalence of knee pain and symptomatic knee osteoarthritis. Annals of Internal Medicine. 2011; 155(11):725-732
- Short A et al. Anatomic study of innervation of the anterior hip capsule: Implication for image-guided intervention. Regional Anesthesia and Pain Medicine. 2018; 43(2):186-192.
- Proliance Orthopedic Associates. (2022). Common shoulder problems. Retrieved from https://www.prolianceorthopedicassociates.com/common-shoulder-problems
- Noriega D et al. A prospective, international, randomized, non-inferiority study comparing an implantable titanium vertebral augmentation device versus balloon kyphoplasty in the reduction of vertebral compression fractures (SAKOS study). The Spine Journal. 2019. doi: 10.1016/j.spinee.2019.07.009.
- Alexandru D and So W. Evaluation and management of vertebral compression fractures. The Permanente Journal. 2012; 16(4):46-51.
- SpineJack system Instructions for Use: 900152 Rev AC.
- VertaPlex IFU (0406-422-715 Rev-E)
- VertaPlex HV IFU (0406-622-715 Rev-AB)
- SpineJack system IFU (900152 Rev-AC)